SEMGLEE® (insulin glargine-yfgn) is a long-acting insulin that works like Lantus®.1 What sets it apart is the company behind it.
Semglee® (insulin glargine-yfgn) is a long-acting insulin that works like Lantus®.1 What sets it apart is the company behind it.
BIOCON BIOLOGICS WILL NEVER STOP
FIGHTING FOR PATIENTS LIKE YOU
DRIVING DOWN THE COST OF INSULIN
by making more affordable options available to patients2
EXPANDING ACCESS TO MORE PEOPLE
with an FDA-approved insulin that can be safely substituted for Lantus3

DELIVERING MORE AROUND THE WORLD
supplying 5.25 billion doses of insulin to patients globally since 20044
AN INSULIN YOU CAN USE WITH CONFIDENCE

It works like Lantus1-3

It's as effective as Lantus1-3

It's as safe as Lantus1-3
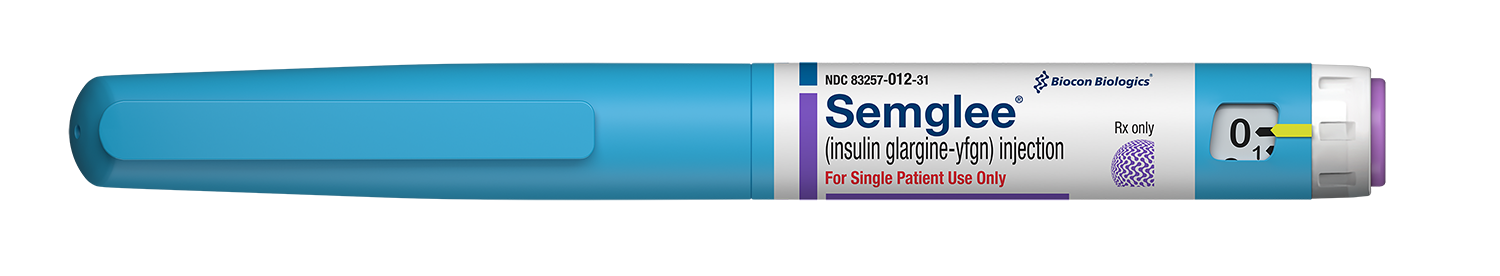

Our product is available as branded SEMGLEE and as an unbranded version of SEMGLEE.
NO CLINICALLY
MEANINGFUL DIFFERENCES
Because SEMGLEE is an interchangeable biosimilar, it's highly similar to the original approved medicine. So you can expect the same familiar experience you've had with Lantus.1,3,5
What's a biosimilar?
While Lantus is a biologic medicine (a medicine that was derived from a living organism), SEMGLEE is an FDA-approved biosimilar, or near-identical copy, of Lantus. Much like a generic drug but more complex in nature.1,3,5
What's an interchangeable biosimilar?
An interchangeable biosimilar is the FDA's designation for a biosimilar that has additional evidence demonstrating that it can be safely substituted for the original biologic medicine at the pharmacy, with the same clinical result expected in any given patient.*5
*Subject to state pharmacy laws.
ONE BIOSIMILAR, TWO WAYS
Ask your doctor about branded SEMGLEE and the unbranded version.
To increase the access and availability of insulin for those who need it most, we made 2 versions of our product: a branded version, SEMGLEE (insulin glargine-yfgn) injection, and an unbranded version, Insulin Glargine-yfgn injection. Both products offer the same high-quality insulin and can be safely substituted for Lantus.1,6
THE UNBRANDED VERSION OF SEMGLEE IS IDENTICAL TO SEMGLEE1,6

SAME overall ingredients

SAME manufacturing process

SAME prefilled device
When you ask for the unbranded version of SEMGLEE, you get the same exact medication as branded SEMGLEE.1,6
WE,RE HERE WITH SUPPORT
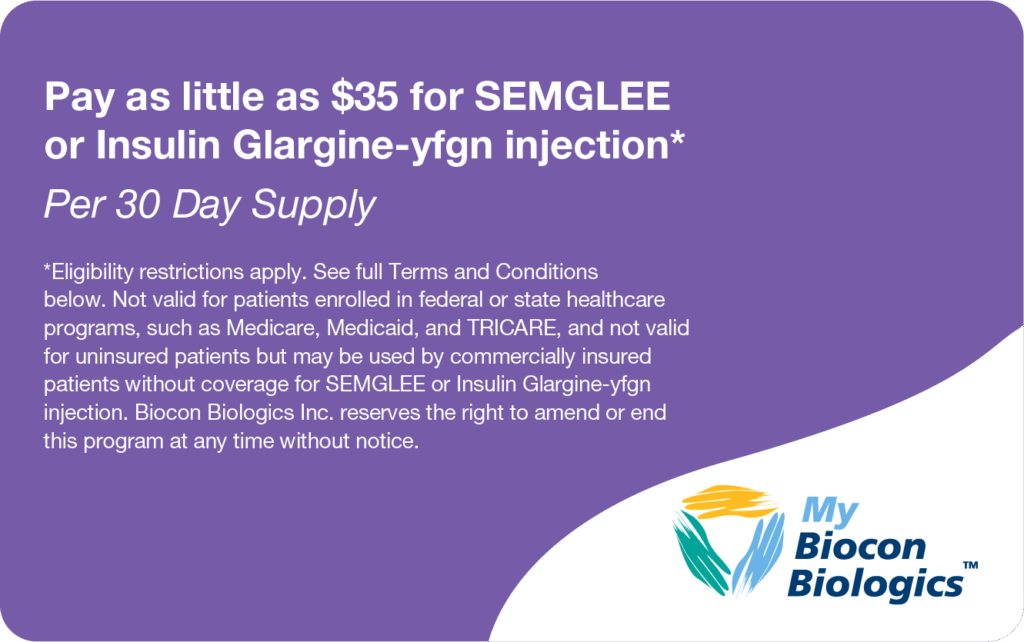
Pay as little as $35
- Biocon Biologics® offers a savings program for SEMGLEE or Insulin Glargine-yfgn injection. This savings cards help eligible patients with commercial health insurance save on out-of-pocket costs
- For questions regarding the Savings Card Program, call 1-800-657-7613 Monday-Friday 8 AM-8 PM ET
†Subject to eligibility requirements. Please see full terms and conditions. Savings may vary depending upon your out-of-pocket costs. Not valid for uninsured patients who are covered by a state- or federally funded healthcare program. Please see full terms and conditions here.
FRIENDLY INJECTION ASSISTANCE
WHEN YOU NEED IT
There’s a lot to learn about SEMGLEE injection. But we know you’ve got this. With our informative injection video, we walk you through every step of the process—so you can learn on your own time, at your own pace.
We,ve been there for you
And we,ve still got your back
Learn more about our global work in insulin here. 
REFERENCES
1. SEMGLEE. Prescribing information. Biocon Biologics Inc; 2025. 2. Joshi SR, Mittra S, Raj P, Suvarna VR, Athalye SN. Biosimilars and interchangeable biosimilars: facts every prescriber, payor, and patient should know—insulins perspective. Expert Opin Biol Ther. 2023;23(8):693-704. 3. FDA approves first interchangeable biosimilar insulin product for treatment of diabetes. FDA News Release. July 28, 2021. Accessed February 7, 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-first-interchangeable-biosimilar-insulin-product-treatment-diabetes 4. Biocon Biologics. Strategic Action. Transformational Growth: Integrated Annual Report FY 2023. Biocon Biologics Limited. Accessed March 18, 2024. https://www.biocon.com/docs/BBL_Integrated_Annual_Report_FY2023_Aug08.pdf 5. US Food and Drug Administration. Biosimilar basics for patients. Last updated October 26, 2023. Accessed February 7, 2024. https://www.fda.gov/drugs/biosimilars/biosimilar-basics-patients 6. Insulin Glargine-yfgn. Prescribing information. Biocon Biologics Inc; 2025. 7. LANTUS. Prescribing information. sanofi-aventis U.S. LLC; 2025.